- NON-CONFORMITY PROCEDURE. This procedure describes the procedure and responsibilities for controlling the identification, documentation, segregation, review, disposition, and notification to affected organizations. Of materials, parts, components, or services which are found to be nonconformance to.
- Showing that the procedure has been updated is not a measure of the effectiveness, that is your verification evidence. For example, if the non-conformance was that a training record for a new employee was not completed, the root cause may be something like “no system in place for new employees to prompt the completion of the training record”.
- Investigating Non-Conformances. As a food auditor an important part of the job is identifying non-conformances during the audit process. Unfortunately, many food businesses struggle with what is required to rectify non-conformances. Any non-conformance identified during an external or internal audit should be seen as an opportunity to really.
Control of Nonconforming Output Procedure
Posted by Integrated Safety Inspection System on 7:29 AM with 2 comments
Procedure is defined and kept in entire organization to ensure that identification and handling of NOK or suspected material is in place to avoid mixing with conform parts. CNC12 The standard defines the exit criteria of the alert production, logistic, maintenance. Epson perfection 660 driver. Alert process is defined on the basis of the severity level of non-conformity. C%2b%2b ostream dev null.
GENERAL REQUIREMENT
This procedure provides guideline to extent information explained in clause 8.7 Control of Nonconforming output of the Quality Manual.The requirement is also enable to provide conformity to clause 8.7.1 and 8.7.2 of Control of nonconforming outputs of ISO 9001:2015 Standard Requirement.
COMMON DEFINITION USED
The definitions addressed are mainly refer to IS0 9000:2015RESPONSIBILITY AND AUTHORITY
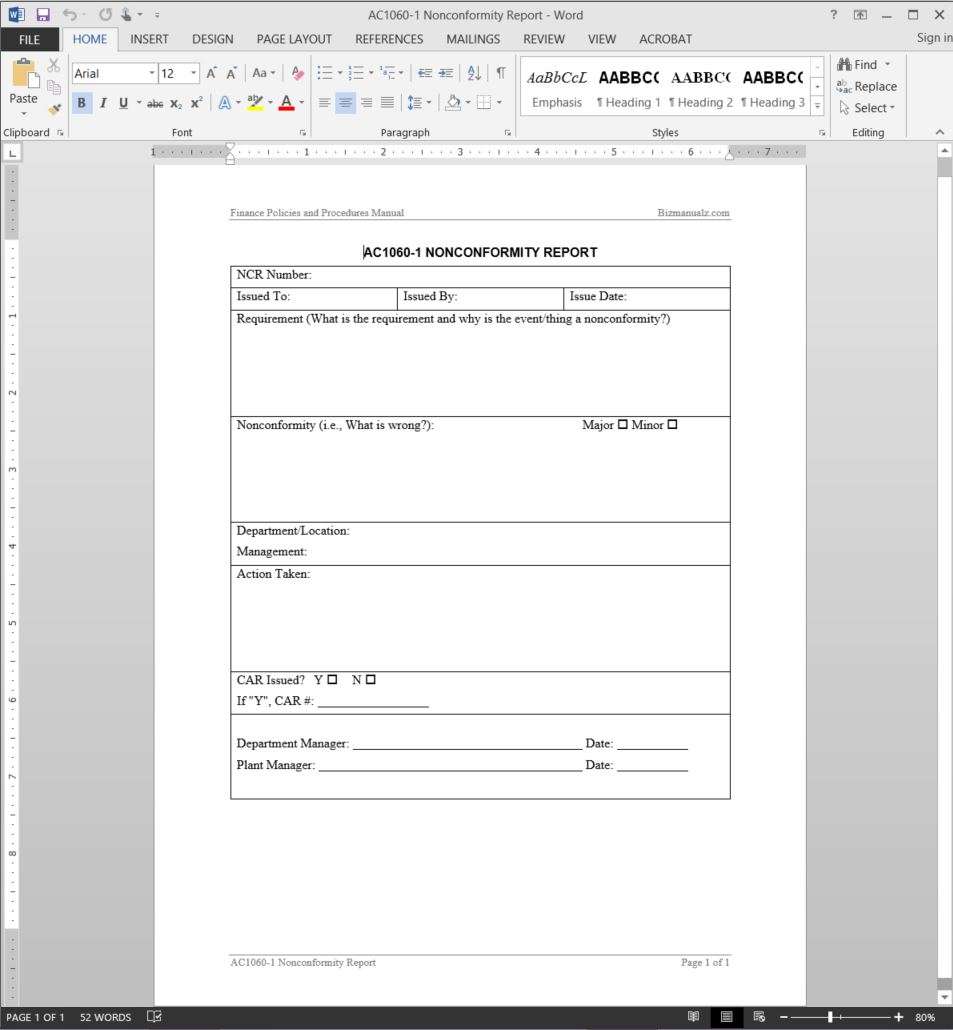
IDENTIFICATION OF NON-CONFORMITY
 Nonconformity of output in the [scope of activity] activity can be identified throughout one or combination of following occurrence;
Nonconformity of output in the [scope of activity] activity can be identified throughout one or combination of following occurrence;Supplier Non Conformance Procedure
- Inappropriate planning as described in clause 8.1 Project Planning of this Quality Manual
- Defect detected by the personnel during construction where it does not meet with specification as defined in clause 8.5.1 Project management control of this Quality Manual
- Absence of competent person if it is required by the clause 7.2 Competence of this Quality Manual
- Defective purchased material or out of specification material as defined in clause 8.4 Control of externally provided processes, products and services of this Quality Manual caused by supplier.
- Other potential undesired consequences associated with services (example; measurement faulty caused by unfit measurement instrument as per clause 7.1.5 Monitoring and measuring resources of Quality Manual)
- Customer complaint
- Complaint received during warranty period
Non Conformance Procedure Sample
CONTROL OF NONCONFORMITY
Non Conformance Procedure Sample
- Whenever nonconformity is detected;
- Nonconformity with regard to the facility or material or physical property, the On-Hold tag must be placed to the defect unit accordingly.
- If related to the human resources, proceed to the clause 7.2 Competence of Quality Manual.
- Review possibility of occurrence due to weaknesses of communication factor as described in clause 7.4 Communication of Quality Manual
- The nonconformity shall be reviewed by
for disposition, which may be any of the following; Hold or must not to be used, or Concession by authorized person The concession of nonconformity is not accepted for concession if high impact to the quality issues or jeopardize the company reputation as defined in Risk Analysis. Results of the review shall be recorded by on the CPAR form The shall be responsible to review the nature and seriousness of nonconformity. For nonconforming condition that have been accepted by concession when regulatory requirements are met. The record of the identity of the person(s) authorizing the concession shall be maintained. If action will be taken other than decision stated in #2, the non-conformity shall be corrected. will verify the measures taken before release non-conformance status. Designated person will decide whether the issuance of CAR form, the nonconformity need further investigation throughout the Corrective Action Procedure or used as record purpose only. For purchased material/part which are found defective or out of specification, [designated person] shall arrange to return the item to the supplier for replacement or further action. CPAR form should be issued. Any defective purchased material requested for concession, the process should follow step as described in the #9. Top management will decide for necessity to inform the nonconformity depends on the severity of the issues. QMR shall maintain CPAR status log. Status of CAR shall be highlighted for management review meeting.